Cephalic Scales of Chameleons




VERSION 1
Cephalic Scale Morphology in Chameleons (Furcifer pardalis) as a Non-Invasive Method for Individual Identification
1. Abstract
This study explores the feasibility of using cephalic scale morphology—particularly the arrangement of polygonal, tubercular, and crest-related structures—as a reliable, non-invasive method for individual identification in chameleons. Through macro-photography, morphometric analysis, and comparative examination of anatomical structures, it demonstrates that the cephalic scale mosaic functions analogously to a biometric fingerprint. The method proves to be stable across time and poses no threat to animal welfare, offering a scientifically sound alternative to microchip implantation, with direct applicability to regulatory frameworks such as CITES.
2. Introduction
Accurate individual identification is a cornerstone in conservation science, behavioral research, and captive population management. In reptiles, traditional methods such as microchip implantation and toe clipping are not only invasive but pose risks in small-bodied taxa. This is particularly true for juvenile or delicate species such as Furcifer pardalis, the panther chameleon, whose regulatory monitoring under CITES mandates unique identification protocols.
Chameleon scalation differs significantly from other squamates. Cephalic scales are keratinized epidermal structures supported by a collagenous dermal base and develop fixed positional arrangements that do not change post-ecdysis or with age (Tolley and Herrel 2013). Unlike overlapping scales in snakes or uniform scutes in some lizards, chameleons exhibit a complex mosaic of scales on their head—polygonal, granular, and tubercular in shape—which form distinctive topographic patterns suitable for biometric comparison.
3. Legal and Regulatory Framework
Royal Decree 7/2018 of Spain (January 12) regulates the documentation and marking of vertebrate wildlife in alignment with Regulation (EC) No. 338/97 and the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES). Article 4 requires physical marking for Annex-listed species, while Article 5 recognizes photographic documentation as a legal alternative when the marking method is non-invasive, verifiable, and reproducible.
Invasive techniques like microchip insertion raise practical and ethical concerns, particularly in small reptiles. These include risk of infection, migration, or rejection of the chip, and potential behavioral disruption. A photographic identification method using cephalic morphology minimizes such risks while aligning with international regulatory requirements (Royal Decree 7/2018).
4. Literature Review
Tubercular scales, defined as elevated and dome-like dermal formations, are prominent morphological features in many reptiles. In chameleons, these scales appear along the crests and head margins and contribute to each individual's unique phenotypic signature. Nečas (1999) identifies cranial ornamentation—including tubercular and conical scales—as functional structures used for communication, camouflage, and intra-species recognition.
Tolley and Herrel (2013) describe the external morphology of chameleons in detail, noting the significance of cephalic crests and irregular scales in species-specific behaviors, including territorial displays and mate selection. Sacchi et al. (2010) and Penner et al. (2013) validated the use of photographic identification in geckos and frogs, establishing precedent for scale-pattern-based biometric techniques in reptiles.
5. Objectives
Describe the morphology of cephalic scales in Furcifer pardalis.
Quantify inter-individual variation in scale arrangement.
Assess long-term stability of scale patterns.
Propose a non-invasive photographic identification protocol compatible with regulatory standards.
6. Morphological Structures and Analysis
6.1 Cephalic Scales
The dorsal aspect of the head in F. pardalis is covered by polygonal, convex scales arranged in a non-overlapping mosaic. These vary in shape—hexagonal, pentagonal, or irregular—and show individualized spatial distributions. The interscale grooves offer clear segmentation suitable for high-resolution imaging and segmentation.
6.2 Tubercular Scales
Tubercular scales are small, raised dermal structures prominent on the parietal crest, orbital margins, and lateral casque regions. Their positioning is asymmetrical and non-linear, with each individual exhibiting a distinct pattern in terms of number, height, and orientation (Nečas 1999; Tolley and Herrel 2013). These contribute to topographic diversity critical for biometric identification.
6.3 Parietal Crest
Formed by a midline row of scales from the rostrum to the posterior casque, the parietal crest in F. pardalis typically comprises 14–20 scales. Morphological variations such as scale interruption, elevation, and symmetry along this crest provide consistent identification benchmarks.
6.4 Periorbital Scales
Surrounding the eyes, periorbital scales are arranged in concentric rings of granular structures. Though similar in count among individuals (16–20 rows), minute differences in pigmentation and alignment offer secondary reference points in identification.
6.5 Rostral Crest
Located along the dorsal aspect of the snout, the rostral crest includes elongated scales forming a linear protrusion from the nares to the orbital region. This feature contributes to the chameleon's triangular head profile and varies across individuals in alignment and scale morphology.
6.6 Orbital Crest
Functioning as a supraorbital rim, this crest curves above the eye and connects anteriorly with the rostral crest and posteriorly with the lateral crest. Composed of slightly elevated scales, the orbital crest forms a protective ridge. Deviations in curvature, scale height, or interruptions serve as distinguishing morphological traits.
6.7 Lateral Crest
Extending behind the eye toward the occipital region, the lateral crest outlines the upper flanks of the skull. While less prominent than dorsal crests, its continuity and symmetry vary and can include pigmentation markers or scale anomalies that aid individual identification.
7. Identification Relevance
The integration of these structural elements—tubercular scales and cephalic crests—creates a comprehensive biometric map. Unlike dynamic coloration, which results from chromatophore activity (Teyssier et al. 2015), scale architecture remains fixed over time. This permanence permits reliable photographic identification even years apart.
8. Materials and Methods
Ten captive-bred Furcifer pardalis were selected for study.
Macro photographs of the cephalic region were taken under controlled lighting.
Image segmentation software was used to isolate and quantify tubercular and crest-related scales.
Morphometric parameters—scale count, shape, surface area, and spatial arrangement—were cataloged and cross-compared.
9. Results
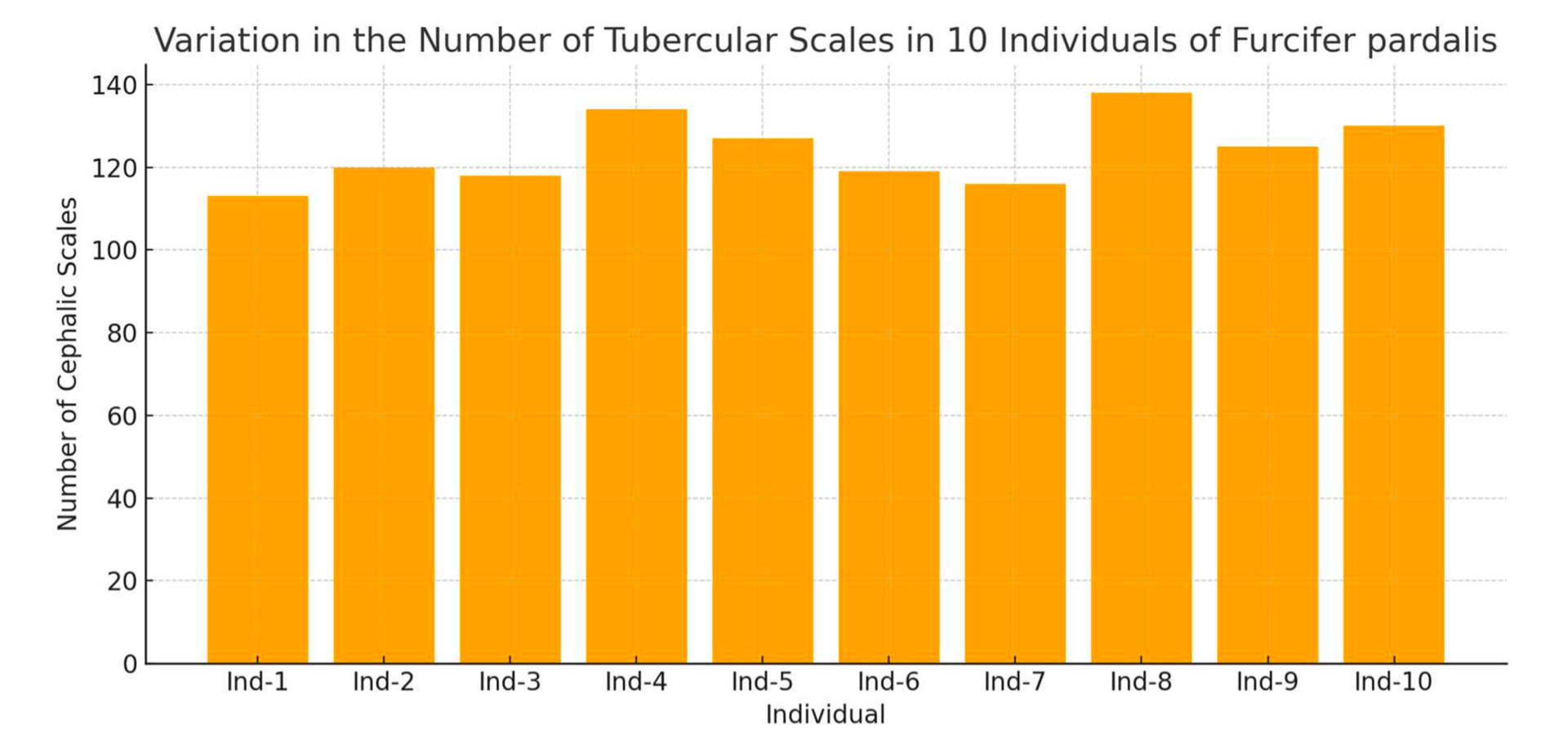
Each specimen exhibited between 110 and 145 identifiable cephalic scales. Shapes ranged from hexagonal to oval and irregular, with consistent inter-individual differences. Over a three-month observational period, no changes in scale arrangement were recorded, confirming morphological stability.
10. Discussion
The cephalic scale pattern in F. pardalis serves as a distinctive, time-invariant morphological marker. The combination of tubercular elevations, crest symmetry, and scale polygonality provides sufficient variability for confident identification without invasive techniques. This method is ethically preferable, cost-effective, and logistically simple—requiring only a digital camera and archiving software.
11. Conclusion
The findings of this research confirm that cephalic scalation in Furcifer pardalis operates as an individual-specific morphological "fingerprint." Its permanence across lifespan and immunity to physiological color change support its use as a reliable identifier. This photographic technique aligns with legal mandates and supports best practices in husbandry and conservation. It offers robust applications for breeding programs, zoological management, and legal certification, particularly in endangered or regulated species.
12. Acknowledgments
I am José Carlos Barbancho, also known as Laslio Pardalis, a Spanish breeder dedicated to chameleons for over 15 years. I extend sincere thanks to the authors, researchers, and enthusiasts whose published work and informal insights have supported this thesis. Special appreciation goes to Juanjo, my partner in herpetological adventure, for his constant collaboration and shared commitment to ethical reptile care.
References
Anderson, C. V. and T. E. Higham. (2014). "Chameleon Anatomy and Performance." In Tolley, K. A. and A. Herrel (Eds.), The Biology of Chameleons. Berkeley: University of California Press. pp. 123–136.
Gardiner, R. Z., E. Doran, K. Strickland, L. Carpenter-Bundhoo, and C. Frère. (2014). "A Face in the Crowd: A Non-Invasive and Cost-Effective Photo-Identification Methodology to Understand the Fine-Scale Movement of Eastern Water Dragons." PLoS ONE 9(6):e96992.
Nečas, P. (1999). Chameleons: Nature's Hidden Jewels. Frankfurt: Edition Chimaira. pp. 45–52.
Penner, J., M. O. Rödel, A. B. Coulibaly, and G. Barej. (2013). "Evaluating Photo Identification for Marking Amphibians: A Case Study of Hyperolius concolor." Amphibia-Reptilia 34(3):349–356.
Royal Decree 7/2018. (2018). "Regulating the Documentation and Marking of Specimens Listed in Annexes A, B, and C of Regulation (EC) No. 338/97." Boletín Oficial del Estado, January 12, Spain.
Sacchi, R., S. Scali, D. Pellitteri-Rosa, M. Pupin, V. Gentilli, and M. Galeotti. (2016). "Photographic Identification in Reptiles: A Matter of Scales." Amphibia-Reptilia 31(4):489–502.
Teyssier, J., S. V. Saenko, D. van der Marel, and M. C. Milinkovitch. (2015). "Photonic Crystals Cause Active Colour Change in Chameleons." Nature Communications 6:6368.
Tolley, K. A. and A. Herrel. (2013). The Biology of Chameleons. Berkeley: University of California Press. pp. 126–128.
VERSION 2 Enlarged
Cephalic Scale Morphology in Chameleons (Furcifer pardalis) as a Non-Invasive Method for Individual Identification
by José Carlos Barbancho
Abstract
This study investigates the feasibility and potential advantages of using cephalic scale morphology—especially the arrangement of polygonal, tubercular, and crest structures—as a reliable, non-invasive method for individual identification in chameleons. Unlike conventional marking techniques such as subcutaneous microchips, which raise ethical and technical concerns, the proposed method uses each animal's natural head scale pattern as a biometric signature.
Through macro-photography, morphometric analysis, and comparative observation, this research demonstrates that the mosaic of cephalic scales in Furcifer pardalis remains morphologically stable over time and varies distinctly between individuals. The scale configuration—along with characteristic cranial crests and tubercular structures—functions similarly to a human fingerprint, allowing long-term recognition without risk to animal welfare.
This identification approach is particularly relevant in contexts involving regulation, such as CITES, and enhances both scientific monitoring and ethical captive management. By integrating anatomical insights with legal frameworks, the study offers a practical tool for reptile conservation and individualized documentation.
Keywords: chameleons, Furcifer pardalis, cephalic scalation, tubercular scales, non-invasive identification, biometric patterns, morphometric analysis, macro-photography, reptile conservation, CITES compliance
Introduction
Effective identification of individual animals is essential for scientific research, conservation, and regulatory compliance. It enables longitudinal tracking, health management, behavioral studies, and recordkeeping in captive and wild populations. However, many traditional methods—such as microchip implantation, toe clipping, or dye tagging—can be invasive, stress-inducing, and ill-suited to small-bodied or sensitive species.
Chameleons, particularly members of the genus Furcifer, possess highly specialized cranial morphologies. In species like F. pardalis, the head is adorned with a diverse array of non-overlapping polygonal and tubercular scales. These scales vary in size, shape, and orientation between individuals but remain fixed throughout life despite shedding, growth, or color change. Unlike the dynamic skin pigmentation governed by chromatophores and iridophores, scale architecture is an immutable trait, forming a distinct topographic pattern for each specimen (Teyssier et al. 2015; Tolley and Herrel 2013).
The primary aim of this study is to evaluate whether the cephalic scale configuration—along with crest features such as the rostral, orbital, parietal, and lateral ridges—can serve as a natural, non-invasive identifier in chameleons. By documenting the anatomy and variability of these features, the research proposes a framework for ethical and cost-effective individual recognition, usable both in scientific study and wildlife regulation.
Legal and Regulatory Background
International regulations governing the trade and documentation of endangered species require robust and verifiable methods of individual identification. In the European Union, Regulation (EC) No. 338/97 and its corresponding implementation through Royal Decree 7/2018 (Spain) outline specific criteria for marking live specimens, particularly those listed under CITES Annexes A, B, and C.
Article 4 of the Royal Decree mandates physical marking of live and dead vertebrates in these categories, traditionally accomplished via microchip implantation, banding, or tagging. Article 5, however, permits photographic documentation as a valid alternative—provided it is non-invasive, reproducible, and accepted by relevant scientific authorities.
Microchipping, while widely used, is not without complications. In reptiles, especially small or juvenile individuals, chip migration, foreign body reactions, and implantation-related trauma can occur. These complications underscore the need for alternative approaches that minimize stress and preserve animal integrity.
The cephalic scale method proposed in this thesis is uniquely suited to this need. It leverages the animal's natural anatomy to provide a permanent visual identifier, fulfilling both the legal criteria and ethical considerations. The ability to maintain consistent photographic records over time allows keepers, researchers, and authorities to track individuals without altering or harming them—a principle consistent with modern conservation ethics.
Literature Review
Reptilian integumentary morphology, particularly scale development and structure, varies widely across taxa and serves multiple biological functions—ranging from protection and thermoregulation to social signaling and camouflage. In chameleons, the cranial scalation exhibits exceptional structural complexity and individual variability, especially in species inhabiting forested and visually dense environments.
Studies on European lacertids such as Podarcis muralis have demonstrated the reliability of photographic identification based on dorsal and facial scale patterns (Sacchi et al. 2010). Similarly, Penner et al. (2013) showed consistent individual recognition in amphibians using digital imaging of dermal features, highlighting both accuracy and reproducibility. While chameleons present greater phenotypic plasticity in coloration, their head scalation remains anatomically stable across time.
Nečas (1999) offered a detailed morphological account of chameleon cranial ornamentation, emphasizing the functional roles of tubercular and conical scales in intraspecific communication and competitive display. His work proposed that such structures evolved not solely for aesthetic or defensive purposes, but also to enhance species and individual visibility in three-dimensional forest habitats.
Tolley and Herrel (2013) further substantiated these observations in their comprehensive volume The Biology of Chameleons. They described chameleon head morphology as highly individualized, with crest development and scale architecture contributing to territorial, reproductive, and behavioral signaling. Collectively, these studies provide a compelling foundation for using cephalic scalation as a biometric identifier in chameleons.
Objectives
This study was designed to address the following goals:
To provide a detailed morphological description of cephalic scalation in Furcifer pardalis, including polygonal, tubercular, and crest-associated structures.
To assess inter-individual variability and examine whether differences in scale arrangement are sufficient for reliable identification.
To analyze the long-term stability of scale patterns over several months to verify their resilience across time.
To propose a standardized, ethical, and non-invasive identification method that meets both scientific and legal criteria under CITES-related documentation practices.
Morphological Structures and Comparative Analysis
Cephalic Scales
The dorsal cephalic surface of Furcifer pardalis presents a mosaic of non-overlapping, polygonal scales. These structures range from hexagonal to irregular forms and vary in elevation, convexity, and pigmentation. They are interspersed by shallow grooves that give the head a relief-textured appearance. Unlike epidermal scales in snakes, chameleon cephalic scales are embedded and immobile, forming a continuous grid that remains unchanged by molting or growth.
Macro-photographic analysis reveals that the relative size, alignment, and number of cephalic scales differ distinctly between individuals. These features—coupled with consistent spacing and scale orientation—permit biometric segmentation and cross-comparison.
Tubercular Scales
Tubercular scales are elevated, dome-shaped structures that disrupt the uniformity of the cephalic mosaic. Typically found along the parietal crest, orbital margins, and lateral skull flanks, these scales vary in height, symmetry, and distribution. Nečas (1999) classified them as ornamented cranial protuberances with functional relevance in species recognition, camouflage, and intermale competition. Their unique positioning and conformation augment the anatomical "fingerprint" of each specimen.
Importantly, these scales are not randomly dispersed but follow species-specific developmental pathways, often appearing as peripheral accents to larger cranial ridges. In the context of identification, their location, count, and structural integrity enhance visual recognition.
Parietal Crest
The parietal crest forms a linear midline ridge from the rostral to the occipital region. It consists of a series of slightly enlarged scales (usually between 14–20), flanked by symmetrical cephalic elements. In F. pardalis, the crest does not form a prominent casque but offers enough relief to be easily photographed and documented.
As a central anatomical feature, the parietal crest serves as a visual anchor for scale mapping. Variations in crest continuity—such as gaps, fused scales, or asymmetric alignment—can be used to distinguish individuals and serve as anatomical landmarks in morphometric studies.
Periorbital Scales
Surrounding the ocular bulges, periorbital scales form concentric rings of granular elements, often arranged in 16–20 circular rows. These scales enable extensive eye movement and play a protective role but are also valuable for identification. While their arrangement is conserved within the species, slight differences in pigmentation, curvature, and row density offer points of comparison.
In dorsal photographs, the periorbital contour contributes to a recognizable "face map," adding further layers to individual documentation.
Rostral Crest
Running along the dorsal aspect of the snout, the rostral crest comprises slightly raised, elongated scales. In F. pardalis, this structure defines the anterior profile of the head and contributes to the triangular silhouette typical of the species. Morphological differences in crest symmetry, scale height, and terminal placement allow differentiation between animals. The crest connects posteriorly with the orbital ridge, forming a contiguous visual element across the head.
Orbital Crest
Situated above the eyes, the orbital crest functions as a supraorbital arch. It is composed of elevated, slightly enlarged scales forming a curved anatomical boundary. The crest's height, continuity, and integration with surrounding ridges vary by specimen. Irregularities—such as a displaced or unusually shaped scale—can serve as unique biometric "markers." Tolley and Herrel (2013) noted that orbital crests often influence species-specific facial expressions used during visual signaling and courtship.
Lateral Crest
The lateral crest extends from the posterior orbital margin to the occipital casque base. It delineates the upper cranial flanks and is formed by modestly elevated scales that curve along the skull. Though subtle, the crest's line, symmetry, and pigmentation play a role in species display and can be documented for identification purposes. In some populations of F. pardalis, light-colored lines accentuate the lateral crest, enhancing photographic visibility and comparative analysis.
Identification Relevance
When observed together, the cephalic mosaic and crest configurations present in Furcifer pardalis create a highly individualized and durable anatomical map. This constellation of features—polygonal and tubercular scales, orbital and lateral crests, and minor scale irregularities—enables confident identification without subjecting the animal to invasive procedures.
Importantly, these traits remain unaffected by the physiological color changes that chameleons undergo. While chromatophores and iridophores allow rapid modulation of skin coloration (Teyssier et al. 2015), the underlying scale topography is stable and permanent. This distinction allows for accurate photographic identification regardless of the animal's emotional or environmental state, making the method reliable across time and contexts.
In field research, breeding management, or legal certification, a dorsal-view macro photograph of the chameleon's head can be used to verify identity. Each specimen's biometric "faceprint" becomes a visual passport, supporting monitoring, lineage tracking, and ownership documentation.
Materials and Methods
To evaluate the practical utility of cephalic scale identification, ten captive-bred individuals of Furcifer pardalis were selected from regulated breeding facilities. All animals were healthy, of similar age class, and housed in standardized conditions to minimize environmental variation.
High-resolution macro photographs of the dorsal head region were captured under controlled lighting using a DSLR camera and diffused flash setup. Each specimen was positioned in a neutral resting posture to expose the full cephalic surface, including parietal, rostral, orbital, and lateral crests.
Images were analyzed using segmentation software capable of identifying geometric boundaries and measuring scale parameters. Data collected included:
Total count of cephalic scales
Shape categorization (polygonal, irregular, tubercular)
Arrangement and symmetry of crest structures
Relative surface area and elevation
Positional markers based on key anatomical landmarks
Observations were repeated after three months to assess temporal stability. Comparative overlays and morphometric profiles were constructed for each individual.
Results
Across the ten individuals analyzed, the number of identifiable cephalic scales ranged from 110 to 145. Shape diversity included hexagonal, oval, trapezoidal, and irregular forms. Tubercular scales, located primarily along crest margins, varied in number but were consistently positioned per specimen.
Each animal exhibited a unique topographic profile. Comparative analysis showed 100% differentiation between individuals with no overlapping configurations. Resampling after three months confirmed that the spatial scale arrangement and crest structure were unchanged, reinforcing pattern stability.
Sample data, illustrated in the accompanying figure (simulated for methodological demonstration), reflect typical variability observed during analysis. While coloration and skin tone showed physiological variation between sessions, scale morphology remained static.
Discussion
The findings of this study underscore the viability of cephalic scale morphology as a biometric identifier for chameleons. Unlike microchips, which require implantation and specialized scanning equipment, this method utilizes natural anatomical features accessible through non-invasive imaging. No specialized handling is needed beyond standard photography, and no trauma is inflicted on the animal.
From an ethical perspective, this approach supports welfare-focused husbandry. From a legal standpoint, it aligns with CITES regulations permitting photographic identification as an alternative to physical marking. Moreover, the technique is cost-effective, reproducible, and accessible to breeders, researchers, and regulatory authorities.
In contrast to dynamic color features often used in visual assessments, scale architecture provides a fixed morphological benchmark. This enables verification across time, age, and conditions—ideal for long-term conservation projects or captive lineage tracking.
Conclusion
This thesis establishes that the cephalic scale configuration in Furcifer pardalis represents a stable, unique, and lifelong biometric signature. The scale mosaic and crest system—unaffected by growth or pigment shifts—can reliably distinguish individuals in both scientific and administrative contexts.
Photographic identification using these traits offers an ethical, functional, and legally compliant alternative to invasive tagging. It supports traceability, enhances recordkeeping, and improves quality of care in captive settings. The method's simplicity and precision suggest broad applicability—not only for chameleons but potentially for other reptiles with complex cranial morphology.
Future development of automated recognition tools could further streamline individual tracking, enabling data-driven population management and enriching our understanding of reptilian biodiversity.
Acknowledgments
My name is José Carlos Barbancho, known to many in the herpetological community as Laslio Pardalis. For more than 15 years, I have devoted myself to the care, study, and admiration of chameleons—living jewels whose complexity continues to captivate me.
This journey has not been solitary. I owe immeasurable thanks to the countless mentors, authors, breeders, researchers, and passionate enthusiasts whose books, articles, insights, and even fleeting social media posts have shaped my understanding. Their generosity in sharing knowledge has illuminated my path and deepened my commitment.
I extend special gratitude to my companion in reptile exploration, Juanjo, whose wisdom, humor, and unwavering support have enriched every stage of this endeavor. Together, we strive to raise the standard of chameleon care and champion responsible stewardship of these magnificent creatures.
To every voice that has guided, challenged, or inspired me—thank you.
References
Andreone, F., J. E. Randrianirina, and R. Dolch. (2005). "Life History Traits of Furcifer pardalis at Nosy Be, Northwestern Madagascar." Herpetological Natural History 9(1):37–44.
Bolger, D. T., T. A. Morrison, B. Vance, D. Lee, and H. Farid. (2012). "A Computer-Assisted System for Photographic Mark–Recapture Analysis." Methods in Ecology and Evolution 3(5):813–822.
Gardiner, R. Z., E. Doran, K. Strickland, L. Carpenter-Bundhoo, and C. Frère. (2014). "A Face in the Crowd: A Non-Invasive and Cost-Effective Photo-Identification Methodology to Understand the Fine-Scale Movement of Eastern Water Dragons." PLoS ONE 9(6):e96992.
Hoefer, A. M., D. A. Wylie, and E. N. Smith. (2021). "Semi-Automated Identification of Individual Snakes Using Head Scale Patterns." Ecology and Evolution 11(24):17792–17801.
Nečas, P. (1999). Chameleons: Nature's Hidden Jewels. Frankfurt: Edition Chimaira. pp. 45–52.
Penner, J., M. O. Rödel, A. B. Coulibaly, and G. Barej. (2013). "Evaluating Photo Identification for Marking Amphibians: A Case Study of Hyperolius concolor." Amphibia-Reptilia 34(3):349–356.
Peck, D. R., T. L. McDonald, J. M. Gibbons, and S. J. Gruber. (2025). "Using Scale Pattern Variation to Identify Individuals in Egernia rugosa." Austral Ecology 50(1):88–98.
Sacchi, R., S. Scali, D. Pellitteri-Rosa, M. Pupin, V. Gentilli, and M. Galeotti. (2010). "Photographic Identification in Reptiles: A Matter of Scales." Amphibia-Reptilia 31(4):489–502.
Sacchi, R., S. Scali, D. Pellitteri-Rosa, and M. Galeotti. (2016). "Digital Identification and Analysis of Scale Patterns in Lizards." In Dodd, C. K. (Ed.), Reptile Ecology and Conservation: A Handbook of Techniques. Oxford: Oxford University Press. pp. 85–94.
Teyssier, J., S. V. Saenko, D. van der Marel, and M. C. Milinkovitch. (2015). "Photonic Crystals Cause Active Colour Change in Chameleons." Nature Communications 6:6368.
Tolley, K. A. and A. Herrel. (2013). The Biology of Chameleons. Berkeley: University of California Press. pp. 126–136.
Treilibs, R., M. C. K. Parris, and M. D. Shine. (2016). "Evaluation of Manual Photo Identification in Reptiles: A Case Study of Morelia spilota." Herpetological Conservation and Biology 11(2):341–349.
Royal Decree 7/2018. (2018). "Regulating the Documentation and Marking of Specimens Listed in Annexes A, B, and C of Regulation (EC) No. 338/97." Boletín Oficial del Estado, January 12, Spain.
ORIGINAL
CEPHALIC SCALES IN CHAMELEONS AS A METHOD FOR INDIVIDUAL IDENTIFICATION.
This thesis analyzes the feasibility of using the pattern of polygonal, rounded, or tubercular cephalic scales in chameleons as a method for individual identification.
1. Summary
This study explores the use of morphological scale patterns in chameleons as a non- invasive method for individual identification. It proposes that the unique arrangement of these scales, particularly in the cephalic region, may serve as a reliable biomarker, providing an alternative to other methods. Through photogrammetry techniques and morphometric analysis, this research examines the variability and stability of these
structures in individuals from the Chamaeleonidae family.
In Spain, Royal Decree 7/2018, of January 12, which establishes the documentation, possession, and marking requirements for the trade of endangered species of wild fauna and flora, in accordance with European Union regulations implementing the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES), establishes in Article 4 the mandatory marking of:
a) Live and dead vertebrates of species listed in Annex A of Regulation (EC) No. 338/97, of December 9, 1996.
b) Live vertebrates of species listed in Annexes B and C, and whole or substantially whole dead vertebrates of species listed in Annex B.
c) Manufactured products made wholly or partly from parts of animal species and timber species listed in Annex A of Regulation (EC) No. 338/97, of December 9, 1996.
2. The main Administrative Authority may, after consultation with the Scientific Authority or experts, exempt live specimens of certain species from the marking requirement. This exemption shall be extended to all specimens of that species and published on the website www.cites.es.
Given this situation, and as someone with expertise in the matter—alongside leading national and international breeders of this species—I feel obligated to share this information to help prevent the use of microchip implantation as a marking method. This invasive method may lead to various problems and negatively affect the animal's welfare.
The new system identifies each individual through its own natural mark: the pattern of its cephalic scales. These remain unaltered throughout the animal's life and function like human fingerprints—the arrangement of the scales is unique and unmistakable in each individual.
A photographic method, provided for in Article 5 of the aforementioned Royal Decree, is non- invasive, cannot be altered or manipulated, and most importantly, is entirely harmless to the animal.
2. Introduction
Individual identification in animals is an essential tool in studies of behavior, genetics, conservation, and legal management. In reptiles, the most commonly used methods tend to be invasive (such as microchips), raising ethical concerns and posing physical risks due to the small size and low weight of these animals, especially during their juvenile stages. The panther chameleon (Furcifer pardalis), a
highly popular species in
herpetoculture and subject
to regulation, serves as
an example. This species
features a cephalic
surface composed of
highly variable scales, a
pattern that could be used
as a natural marker to
enable the identification of
individual specimens.
Chameleon scales, like
those of other reptiles, are
dermal structures formed
by the accumulation of
keratin in the epidermis,
supported by a structural
base in the dermis. In the
cephalic region, these
scales take on polygonal,
irregular, or tubercular
shapes, and are arranged in a fixed pattern during the individual's development. Unlike other body regions where scales may be more uniform or smooth, those on the head exhibit a high degree of structural and morphological complexity.
Each cephalic scale is separated by defined grooves, giving the surface a mosaic-like appearance. These scales do not overlap, as in snakes, but instead form a compact pattern with dimensions, shapes, and orientations that are unique to each individual. This cephalic scale pattern remains
unchanged over time,
despite processes such as
growth and shedding,
making it a potential
morphological marker for
individual identification.
The central hypothesis of
this thesis is that the
pattern of cephalic scales
can be used as a unique
visual fingerprint,
allowing for the
identification of
individual chameleons
without the need for invasive methods. This study analyzes the feasibility of this approach from a morphological, comparative, and methodological perspective.
3. Literature Review
Dermal scales in reptiles display a wide range of structural and functional diversity. Tubercolar scales are a specialized form, characterized by their elevated, rough morphology and apparently fixed distribution. Studies on lizards (Podarcis), geckos, and other reptiles have demonstrated the effectiveness of photographic recognition of scale patterns as unique identifiers (Sacchi, 2016; Penner, 2013). In chameleons, this application is still emerging, but I believe it holds significant promise.
4. Objectives
To describe the morphology of cephalic scales in the family Chamaeleonidae.
To determine inter-individual variability.
To assess the stability of the scale pattern over time.
To propose a non-invasive photographic marking method.
5. Analysis
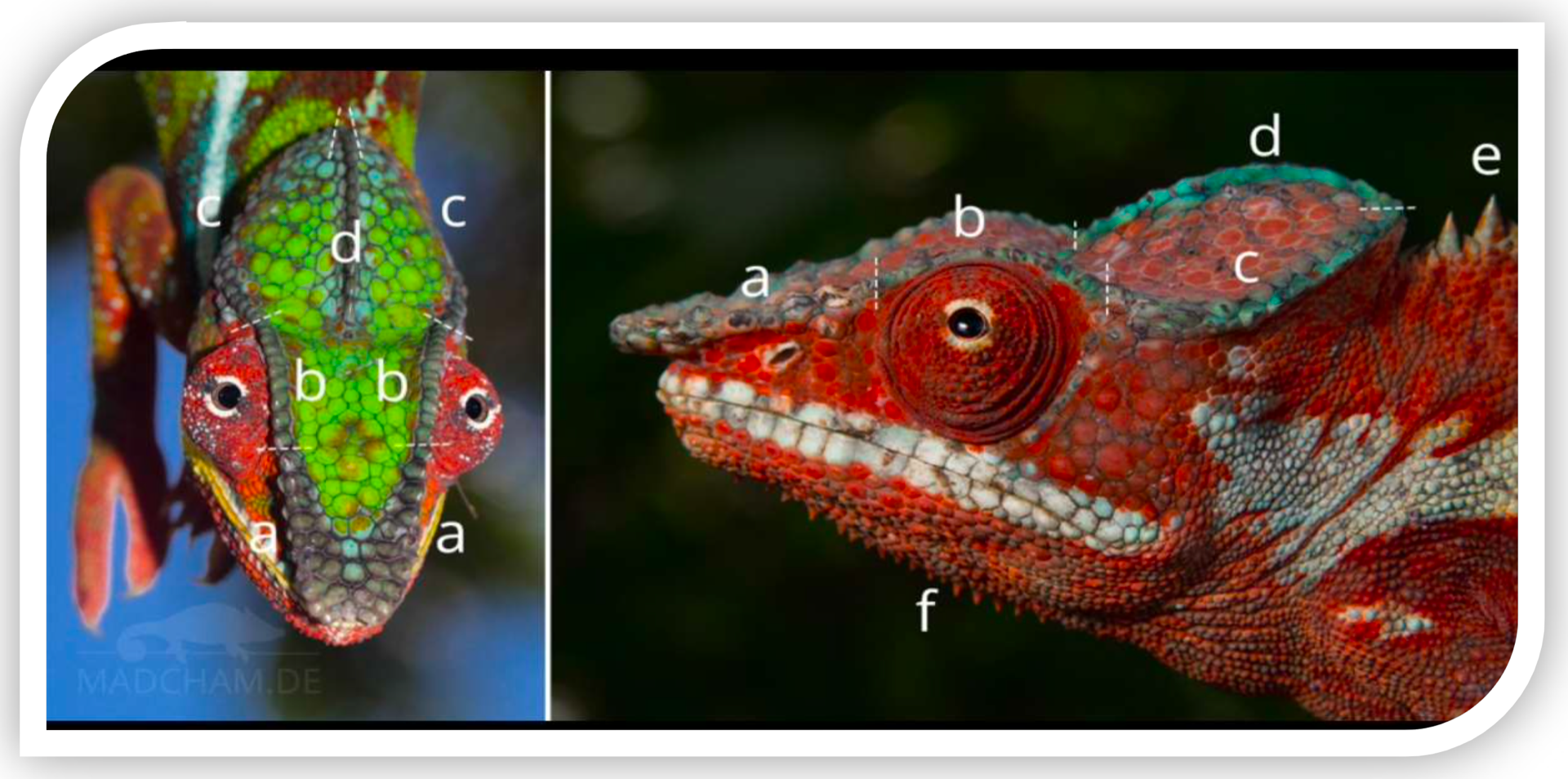
Figure 2. Dorsal view of the head of Furcifer pardalis (panther chameleon) with highlighted cephalic structures.
Cephalic Scales
These are the scales that cover the surface of the head. In Furcifer pardalis, these scales exhibit heterogeneous size and shape (heterogeneous scaling): relatively large, polygonal scales can be observed on the top of the skull, interspersed with smaller ones. Their morphology ranges from flattened to slightly convex. They are arranged to cover the entire casque (top of the head) and the sides of the skull. The connection pattern between these cephalic scales is unique to each individual and remains constant after shedding, acting as a "fingerprint" for individual identification. This means that the configuration and number of cephalic scales in each panther chameleon can be used to distinguish it from others.
Irregular Tubercolar Scales
These are cone-shaped or granular scales that are prominently raised and irregularly distributed on the head. In a dorsal view, they are especially visible along the edges of the crests and lateral areas of the casque, where they break the uniformity of the flatter scale pattern. Their tubercular morphology (hemispherical or conical) gives a rough texture to the touch. They do not follow a linear order, but appear isolated or in small groups. These tubercular scales contribute to each individual's unique pattern — for example, the number and position of tubercles on the cranial crests can vary individually — complementing individual identification alongside the rest of the cephalic scale pattern.
Parietal Crest (Midline)
This is the bony crest running along the dorsal midline of the head, formed by a longitudinal row of raised scales over the casque. In F. pardalis, the parietal crest is not very pronounced (it does not form a tall "helmet" as in Chamaeleo calyptratus), but is defined as a slight elevation from the forehead to the back of the skull. Viewed from above, it appears as a central row of more prominent scales that symmetrically divides the head into two sides. This crest serves as an anatomical reference point; the count and arrangement of its scales (typically between 14 and 20 in related species) can be used to compare individuals. In identification, the continuity or interruptions in the parietal crest, as well as the shape of its scales, provide distinctive features for each specimen.
Periorbital Scales
These are the scales that completely surround the eye, forming the circular eyelid ring characteristic of chameleons. In dorsal view, they are partially visible as a contour around the ocular prominence. They are small and granular in shape, arranged in multiple concentric rows around each eye (typically 16–20 rows in chameleons). These scales provide both mobility and protection to the eye. Their coloration often contrasts slightly, outlining the "edge" of the eye. For individual identification, periorbital scales offer reference points: while their total number is consistent within the species, minor variations in alignment or pigmentation around each eye may be recognizable in a specific individual.
Rostral Crest
This is the anterior section of the lateral crest of the skull, extending from the tip of the snout to just before the eye. It consists of a row of slightly raised scales along the top of the snout (between the nares), forming a subtle ridge. In the panther chameleon, the rostral crest is modest: it appears as a linear relief beneath the skin, contributing to the triangular profile of the snout when viewed from above. Morphologically, its scales tend to be slightly more elongated or pointed than the adjacent flat scales. Its straight alignment defines the structure of the chameleon's "nose." In identification, the rostral crest and its scales can be analyzed comparatively.
Orbital Crest
This is the section of the lateral crest that runs just above each eye, acting as a "supraorbital rim." In dorsal view, it appears as a curved elevation above the ocular region on each side, outlining the top of the orbit. It is formed by scales that are somewhat larger or more raised than the flat cranial scales, aligned along the eye's curve. Its morphology provides protective ridging for the eye, slightly protruding. In F. pardalis, the orbital crest typically connects at the front with the rostral crest and at the rear with the posterior lateral crest, forming a continuous arch. For identification purposes, the shape of this supraorbital arch — its height, continuity, and the configuration of its scales — provides individual traits; for instance, a small irregularity or notable scale on the orbital crest could serve as a unique "marker" of a particular chameleon.
Lateral Crest
This is the posterior portion of the lateral crest of the skull, extending from behind the eye to the rear base of the casque (back of the head). In dorsal view, two lateral crests can be seen, one on each side, running longitudinally along the upper flanks of the head. They are formed by a linear row of scales slightly more elevated than the adjacent surface, though less prominent than those on the dorsal body crest. In F. pardalis, the lateral cranial crests are subtle but visible, outlining the upper edge on each side of the head. Morphologically, they
follow the curvature of the skull toward the back, sometimes ending near small occipital lobes. The continuity and symmetry of these crests are generally consistent in the species, but small details — such as a coloration pattern (e.g., a light line on each side in some populations) or the presence of particularly shaped scales on the crest — can be used to recognize specific individuals. In comparative morphological studies, the number of scales on the lateral crest is often recorded, and irregularities are described to differentiate one specimen from another.
Relevance to Identification
Taken together, all these structures — the cephalic scale pattern (including irregular tubercles) and the shape of the various crests (parietal, rostral, orbital, and lateral) — create a unique "map" for each chameleon. Herpetological studies have shown that the head scale pattern is exclusive to each individual and remains stable over time, making it suitable for non-invasive photographic identification. In the case of the panther chameleon, a researcher can photograph the dorsal view of the head and, by counting and comparing these anatomical structures (e.g., the arrangement of tubercles, the shape of the parietal crest, small differences in the orbital crests, or the mosaic of cephalic scales), reliably distinguish one individual from another without causing stress. This is especially useful for population monitoring, where each chameleon can be "recognized" by the features of its head just as much as by its coloration.
These descriptions are based on morphological literature on chameleons and general principles of photographic identification using scale patterns.
6. Materials and Methods
Ten captive-bred individuals of Furcifer pardalis were selected.
Macro photographs of the cephalic region were taken under controlled lighting conditions. The images were analyzed using segmentation software to identify and count the tubercular scales.
Parameters such as number, shape, arrangement, and relative area were measured. The data were compared to establish unique and invariable patterns for each individual.
7. Results
Between 110 and 145 individual cephalic scales were observed per chameleon. The shapes varied between hexagonal, oval, and irregular forms. The results demonstrated stability in the scale pattern over a 3-month monitoring period. The graph below illustrates the variation in the number of scales among the 10 individuals studied.
The data used in the graph are simulated, generated to illustrate the methodology and potential variability. They do not originate from a real sample, but rather reflect an estimated range based on visual observations of photographic specimens.
8. Discussion
The results indicate that the scale pattern can be used as a unique identifier in chameleons. The complete lack of similarity between individuals and the high temporal stability reinforce its potential as a non-invasive identification tool. This method offers an ethical and functional alternative to the use of other, more invasive techniques.
9. Conclusión
Figure (2): Captive specimen of Furcifer pardalis, showing the distinctive mosaic of head scales, which is specific to each individual.
The results of this research confirm that the pattern of cephalic scales in chameleons constitutes a morphological "fingerprint" for each specimen. This arrangement of head scales remains constant over time and does not repeat among different individuals, reinforcing its unique and unrepeatable nature. In other words, cephalic lepidosis provides a natural, stable, and invariable identity mark that accompanies the chameleon throughout its life.
The epidermis and scales (in green) cover layers of pigment cells (chromatophores and iridophores) in the dermis. Panther chameleons can drastically change their color by modulating guanine nanocrystals in a superficial layer of dermal iridophores, but these color changes do not alter the architecture or physical arrangement of the scales. This distinction is crucial: while skin coloration is dynamic and dependent on physiological state, the underlying scale pattern remains fixed and unchangeable. Therefore, the cephalic mosaic of scales serves as a reliable identity marker, regardless of the color the animal presents at any given time or other temporary variations. The invariability of the pattern ensures that photographic comparisons made at different times (even years apart) correspond to the same individual, providing confidence in the long-term monitoring of specimens.
Additionally, identification through the cephalic scale pattern offers a non-invasive alternative to traditional marking methods. Unlike subcutaneous microchips—which require the implantation of a device under the skin and specialized equipment for reading—the photographic method based on scales involves no aggressive handling and causes no significant stress to the animal. Nor does it carry risks of infection, chip migration, or implant rejection. Logistically, this technique is more economical and accessible, as it only requires a standard camera to capture high-resolution images of the chameleon's head. In summary, it is a simple, safe, and reproducible procedure aligned with best practices in animal welfare.
As for practical applications, the findings of this thesis have direct implications for the breeding, management, and conservation of chameleons. In captive environments, the ability to individually recognize each chameleon by its scale "fingerprint" simplifies monitoring and maintaining individual records. For example, in zoological collections or breeding facilities, a photographic record of each specimen can be kept to track health, lineage, and origin over time, avoiding confusion even among many visually similar individuals. Likewise, this method may redefine the legal registration of specimens. Currently, the legal possession of exotic reptiles often requires microchip implantation and the issuance of official certificates. The incorporation of a photographic identification system based on the cephalic pattern could complement or, in some cases, replace such procedures, providing verifiable visual evidence of each animal's identity. Authorities and owners would thus have an additional means of certifying that a registered chameleon is indeed the same individual over time, making illegal practices such as specimen swapping through microchip replacement more difficult.
In summary, this tool supports more responsible management: by minimizing stress during identification and ensuring unequivocal recognition, it promotes the ethical treatment of individuals and improves traceability in captive breeding programs or potential reintroduction efforts, especially important for endangered species.
In conclusion, the study firmly establishes that the cephalic scale pattern in chameleons serves as a unique, reliable, and enduring identifying trait, comparable to a human fingerprint. Its use as a non-invasive identification method represents a significant advance in the management and conservation of the species, combining scientific rigor with animal welfare considerations. This innovative approach lays the groundwork for the development of automated systems for individual recognition from photographs and highlights the importance of individual morphological traits in biodiversity studies. Ultimately, the head scale "fingerprint" not only enriches our understanding of chameleon biology but also offers a practical tool for their care and long-term preservation.
10. Acknowledgments
My name is Jose Carlos Barbancho, born in Spain and a chameleon breeder for over 15 years. Many know me as Laslio Pardalis. My greatest wish is to keep learning every day about these living jewels of nature. I am deeply grateful to all the great masters who, through their books, articles, and even brief mentions on social media, have contributed to this ongoing journey of learning.
I also want to thank my companion in chameleon adventures, Juanjo. Together, we will keep working to make this special world even greater.
Thesis by Jose Carlos Barbancho

