Soft Bodies, Hard Consequences: Nutritional and Toxicological Risks of Hornworms and Silkworms in Chameleon Diets

Moths and Butterflies of Europe and North Africa
www.leps.it





Silkmoths and more: The true silkworms: Bombyx mori
silkmothsandmore.blogspot.com

Not all that glitters is gold—when palatability masks pathology
Abstract
Hornworms (Manduca sexta) and silkworms (Bombyx mori) are widely used as feeder insects in captive reptile husbandry, particularly for chameleons. While their soft bodies and high moisture content offer hydration benefits, their nutritional and physiological profiles reveal significant limitations. This article critically examines ten key concerns, including calcium–phosphorus imbalance, digestive obstruction, poor gut-loading efficiency, behavioral conditioning, and risks of hyperhydration. It also addresses the toxicological implications of bioactive compounds such as DNJ and synthetic dyes, and proposes the use of adult moths as biologically superior alternatives. Drawing on comparative anatomy, metabolic physiology, and verified toxicology, the findings underscore the need for evidence-based feeding protocols and caution against the overreliance on caterpillars as staple feeders. Practical recommendations are provided to support safe, nutritionally balanced, and ecologically valid chameleon care.
Keywords
Chameleon nutrition; hornworms; silkworms; feeder insects; calcium–phosphorus ratio; gut-loading; impaction; peristalsis; DNJ toxicity; hyperhydration; insect metamorphosis; moths; reptile husbandry; dietary rotation; synthetic dyes
Introduction
Chameleons (Chamaeleonidae) are among the most frequently kept reptiles in private and institutional collections. Their popularity stems from their striking morphology, color-changing ability, and behavioral complexity. However, their dietary requirements in captivity pose significant challenges. In the wild, chameleons consume a wide diversity of arthropods, including orthopterans, coleopterans, dipterans, and arachnids (Tolley & Herrel, 2013). In captivity, however, the range of available feeder insects is limited to those that can be mass-produced economically and hygienically. The most commonly available feeder insects include Acheta domestica (house cricket), Gryllus bimaculatus (field cricket), Blaptica dubia (Dubia roach), Zophobas morio (superworm), Tenebrio molitor (mealworm), Hermetia illucens (black soldier fly larvae and flies), Manduca sexta (hornworm), and Bombyx mori (silkworm).
The commercial availability of Manduca sexta and Bombyx mori as feeder insects emerged in the early 2000s, with widespread distribution beginning around 2005–2007 (Finke, 2013). Their introduction was a positive development for herpetoculture, offering feeder options with high moisture content and relatively low fat. Chameleons, which are prone to dehydration and renal complications in captivity, benefit from the hydration provided by these larvae.
Biology of Manduca sexta
The hornworm, Manduca sexta, is the larval stage of the Carolina sphinx moth. Native to the Americas, it is a specialist herbivore on Solanaceae plants, particularly Nicotiana tabacum (tobacco) and Solanum lycopersicum (tomato). The larvae are large, soft-bodied, and grow rapidly, reaching lengths of up to 10 cm. Their high water content (~85%) and low fat profile make them attractive as feeder insects (Finke, 2013). However, their natural diet includes plants rich in alkaloids such as nicotine and solanine, which they can sequester and excrete through spiracles as a chemical defense (Kumar et al., 2014).
Biology of Bombyx mori
The silkworm, Bombyx mori, is the domesticated larval form of the silk moth. It is an obligate feeder on Morus alba (white mulberry) leaves and has been bred for thousands of years for silk production. Silkworms are soft-bodied, slow-moving, and contain similarly high moisture content (~82–85%) with low fat and sugar levels (Finke, 2013). Unlike hornworms, silkworms do not sequester plant toxins, though their gut contents may retain phytochemicals such as 1-deoxynojirimycin (DNJ), a glucosidase inhibitor found in mulberry leaves (Kimura et al., 2007).
Nutritional Benefits
Both hornworms and silkworms offer hydration benefits due to their high water content. Their dry matter composition is comparable to other feeder insects, with protein levels around 9–15% and fat levels below 5% (Finke, 2013). They are low in carbohydrates and do not contain excessive sugars, making them suitable for species prone to metabolic disorders. However, their mineral and vitamin content is highly variable and depends on the composition of their diet during rearing. Commercial breeding operations often use proprietary artificial diets, and the exact nutrient profiles are rarely disclosed. This lack of transparency necessitates caution when evaluating the true nutritive value of these feeders.
Risks of Feeding Hornworms
Wild hornworms pose significant risks due to their natural diet. When feeding on Solanaceae plants, they can accumulate toxic alkaloids such as nicotine, solanine, and tomatine. These compounds are known to cause neurotoxicity, hepatotoxicity, and gastrointestinal distress in vertebrates (Yildiz et al., 2013). In chameleons, ingestion of wild hornworms may lead to symptoms such as lethargy, vomiting, anorexia, and long-term organ damage. Nicotine, for example, has an LD₅₀ of 50 mg/kg in rats and is known to cause tachycardia, tremors, and respiratory failure (Benli, 2024).
Captive Breeding of Hornworms and Diet Composition
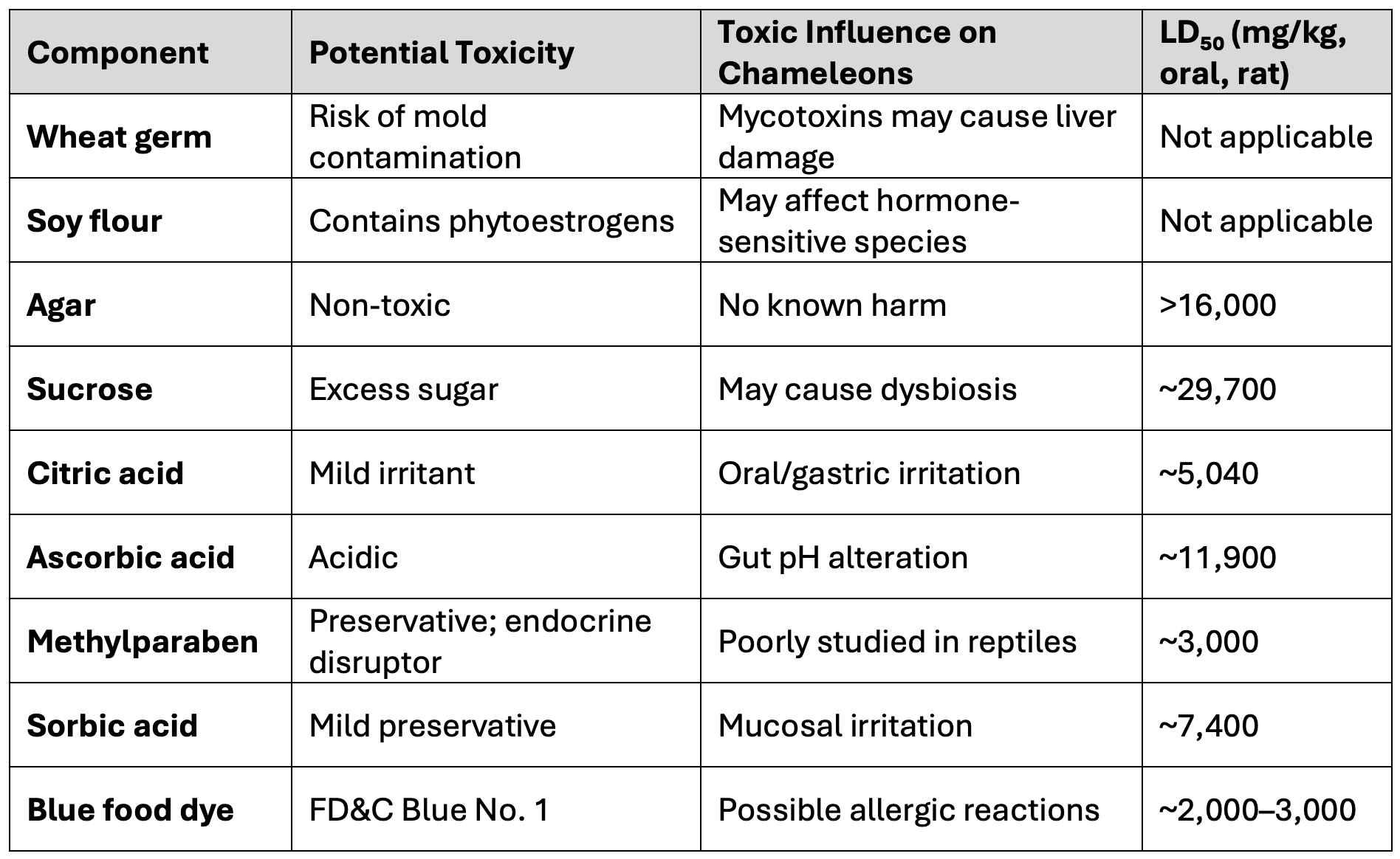
Commercially bred hornworms are typically raised on artificial diets composed of wheat germ, soy flour, agar, sucrose, citric acid, ascorbic acid, methylparaben, sorbic acid, and food dye. While these ingredients are generally safe, some carry mild toxicity risks. The exact formulations are proprietary, and companies do not disclose their feed compositions, citing commercial confidentiality. This opacity introduces uncertainty regarding the safety and consistency of the feeders.
Below is a summary table of common diet components and their toxicological profiles:
The Azure Curse: Why Are Captive Hornworms Blue?
Wild Manduca sexta larvae are typically bright green, a coloration that provides camouflage among the foliage of their host plants, primarily within the Solanaceae family. This green pigmentation is the result of a combination of dietary chlorophyll intake and endogenous biliverdin synthesis, which together produce the characteristic hue (Grill & Götz, 1992).
In contrast, captive-reared hornworms are often turquoise or blue. This color change is not genetic but entirely diet-induced. Commercial hornworm chow, an artificial gel-based diet, contains FD&C Blue No. 1 (Brilliant Blue FCF), a synthetic food dye added deliberately during formulation. The dye is absorbed into the larval cuticle and hemolymph, replacing the green pigmentation that would otherwise result from chlorophyll-rich plant consumption.
The inclusion of blue dye serves several practical purposes:
- Visual Differentiation: It allows breeders and consumers to distinguish captive-reared hornworms from wild-caught individuals, which may carry toxic alkaloids from Solanaceae plants.
- Quality Control: Uniform coloration indicates consistent diet consumption and health status across the colony.
- Marketing and Safety: The blue coloration signals that the worms have not fed on potentially toxic plants, reassuring reptile keepers of their suitability as feeders.
FD&C Blue No. 1 is approved for use in animal feed and has a relatively low toxicity profile. Its LD₅₀ in rats is approximately 2,000–3,000 mg/kg (Borzelleca et al., 1990). While generally considered safe, its long-term effects in reptiles are not well studied, and anecdotal reports of allergic reactions in mammals suggest that caution is warranted in sensitive species.
For a chameleon weighing 150 grams, the estimated LD50 range would be 300 to 450 milligrams. Based on this, the number of hornworms required to reach LD50 thresholds is as follows:
At the low end (300 mg), approximately 60 hornworms
At the high end (450 mg), approximately 90 hornworms
Subclinical concern thresholds can be estimated at 10 percent of LD50: 30 to 45 milligrams of dye Equivalent to 6 to 9 hornworms
Feeding a chameleon 6 to 9 dyed hornworms in a short period may approach subclinical concern thresholds, especially if repeated weekly. Given the lack of reptile-specific metabolic data, this exposure could plausibly affect hepatic or renal function over time.
FD&C Blue No. 1 is not nutritionally necessary for hornworms and has not been evaluated for safety in chameleons. While acute toxicity is unlikely at typical feeding levels, cumulative exposure may pose risks. Until reptile-specific toxicological studies are available, dyed hornworms should be used sparingly, and preference should be given to larvae raised on natural plant diets.
It must be said clearly: feeding dyed hornworms to chameleons is not just unnecessary, it isdangerous and irresponsible. If 6 to 9 worms may approach toxic thresholds, then even 3 to 5 could already trigger mild, invisible reactions. These are not theoretical risks. They are plausible, measurable, and entirely avoidable. The dye serves no nutritional purpose and introduces a chemical burden that has never been studied for reptile safety. Would you feed your child a substance that was never tested for their biology just because it looks neat? Then why risk it with your animals? Stop feeding dyed hornworms. There is no benefit, and there is real danger.
While FD&C Blue No. 1 (Brilliant Blue FCF) is widely used in human food products and considered low-risk in regulated contexts, its application in captive hornworm diets introduces a set of biological and ethical concerns that remain largely unexamined. Reptiles, including chameleons, are routinely fed dyed hornworms without any formal toxicological evaluation of the dye's safety in non-mammalian vertebrates. This practice is driven by aesthetic convenience rather than nutritional necessity, and it operates in a regulatory vacuum.
The following five chapters examine critical dimensions of this issue
1. Metabolic Pathway Unknowns in Reptiles
The metabolic fate of FD&C Blue No. 1 (Brilliant Blue FCF) in reptiles remains entirely uncharacterized, presenting a significant gap in toxicological understanding. In mammals, this dye is primarily excreted unchanged via feces, with minimal hepatic transformation (EFSA, 2010). However, reptiles possess markedly different hepatic enzyme systems, particularly in the cytochrome P450 family, which governs Phase I detoxification. Hanioka et al. (2008) demonstrated that squamates exhibit reduced activity in several CYP isoforms compared to mammals, suggesting slower clearance rates and potential for bioaccumulation of xenobiotics.
This divergence in enzymatic function implies that reptiles may metabolize synthetic dyes more slowly or inefficiently, increasing systemic exposure duration. Furthermore, reptiles often lack robust Phase II conjugation pathways, such as glucuronidation and sulfation, which are critical for rendering compounds water-soluble and excretable. Without these mechanisms, Brilliant Blue FCF may persist in tissues longer than anticipated, potentially interacting with cellular structures or interfering with normal physiological processes.
Given that FD&C Blue No. 1 has never been evaluated in reptilian models, extrapolating safety thresholds from rodent LD₅₀ data is scientifically unsound. The assumption of linear scaling across taxa ignores fundamental biochemical differences. Until targeted pharmacokinetic studies are conducted in reptiles, any exposure to synthetic dyes must be considered speculative and potentially hazardous.
2. Impact on Gut Microbiota
The gut microbiota of reptiles plays a central role in digestion, immune modulation, and nutrient assimilation. In insectivorous species such as chameleons, microbial communities assist in breaking down chitin, regulating pH, and synthesizing essential metabolites. Disruption of this delicate ecosystem can have cascading effects on health, including malabsorption, inflammation, and increased susceptibility to pathogens.
In mammalian models, synthetic food dyes have been shown to alter gut microbial composition. Sharma and Gautam (2015) reported that FD&C Blue No. 1 and other azo dyes reduce populations of beneficial bacteria such as Lactobacillusand Bifidobacterium, while promoting opportunistic strains. These shifts are associated with increased intestinal permeability and systemic inflammation. Although no equivalent studies exist for reptiles, their slower gastrointestinal transit and reliance on microbial fermentation suggest heightened vulnerability to chemical perturbation.
Moreover, reptiles often exhibit temperature-dependent digestion, meaning that any disruption to microbial balance may be amplified under suboptimal thermal conditions. The introduction of FD&C Blue No. 1 into the diet via dyed hornworms could therefore impair microbial homeostasis, leading to subtle but chronic health declines. Without empirical data on dye–microbiota interactions in reptiles, caution is warranted.
3. Behavioral and Neurological Effects
Synthetic dyes such as FD&C Blue No. 1 have been implicated in behavioral and neurological changes in mammals, particularly in juvenile populations. Borzelleca et al. (1990) documented increased locomotor activity and altered neurotransmitter levels in rats exposed to high doses of Brilliant Blue FCF. In human studies, similar dyes have been associated with hyperactivity and attention deficits, prompting regulatory scrutiny in pediatric nutrition.
Reptiles possess homologous neurochemical systems, including dopaminergic, serotonergic, and GABAergic pathways, which regulate feeding behavior, stress response, and thermoregulation. While no direct evidence links FD&C Blue No. 1 to behavioral effects in reptiles, the potential for neurotoxicity via oxidative stress or excitotoxic mechanisms cannot be dismissed. Ratajczak et al. (2013) noted that synthetic dyes can cross the blood–brain barrier in mammals, suggesting a plausible route of action in other vertebrates.
In chameleons, which are highly sensitive to environmental and dietary changes, even low-level neurochemical disruption could manifest as altered feeding patterns, reduced responsiveness, or impaired motor coordination. The absence of reptile-specific neurotoxicity data does not imply safety—it highlights a critical area of neglect. Until such studies are conducted, the behavioral risks of dyed feeders must be considered real.
4. Regulatory Silence
FD&C Blue No. 1 is approved for human consumption under strict dosage limits, but its use in feeder insects for exotic pets falls outside the scope of current regulatory frameworks. The U.S. Food and Drug Administration (FDA), the European Food Safety Authority (EFSA), and the Joint FAO/WHO Expert Committee on Food Additives (JECFA) have not issued guidance on synthetic dye exposure in non-mammalian vertebrates. This regulatory silence allows commercial breeders to introduce untested compounds into the food chain without oversight or disclosure.
EFSA's 2010 re-evaluation of Brilliant Blue FCF emphasized its low absorption and rapid excretion in humans, but explicitly noted that safety data in other taxa were lacking. Despite this, dyed hornworms are widely sold and fed to reptiles, often without informing consumers of the chemical additives involved. Pet owners may unknowingly expose their animals to substances with unknown toxicological profiles, relying on visual cues rather than nutritional or safety data.
The absence of regulation does not equate to safety. It reflects a blind spot in toxicological governance, where aesthetic convenience overrides biological integrity. Until regulatory bodies address this gap, dyed feeders must be treated as unverified and potentially unsafe.
5. Ethical Breeding Practices
The use of synthetic dyes in hornworm rearing is driven by aesthetic and logistical convenience, not by nutritional or physiological necessity. Dyed larvae are easier to identify and distinguish from wild specimens, which may carry plant-derived toxins. However, this practice raises ethical concerns about the priorities of commercial breeders. If visual uniformity is achieved at the expense of animal safety, then the practice is indefensible.
Ethical breeding should prioritize the biological integrity of both the feeder and the consumer. This includes transparency in husbandry practices, disclosure of chemical additives, and avoidance of unnecessary exposure to untested compounds. The inclusion of FD&C Blue No. 1 in hornworm diets serves no purpose beyond visual standardization. It introduces a chemical burden that has never been evaluated for safety in reptiles, and may pose real risks, especially with repeated feeding.
Pet owners trust breeders to provide safe, biologically appropriate food for their animals. When that trust is violated through undisclosed chemical manipulation, the consequences fall on the animals—often silently, through subclinical damage. Ethical responsibility demands that dyed hornworms be phased out until their safety is proven. Anything less is negligence.
Feeding of Silkworms in Captivity
Silkworms (Bombyx mori) are typically fed either fresh Morus alba leaves or a processed mulberry-based chow composed of dried leaf powder, wheat germ, cellulose, and stabilizers. This diet supports rapid larval growth and silk production. However, mulberry leaves contain a range of bioactive compounds that are not fully metabolized or excreted by the larvae prior to ingestion by predators. Notably, some hornworm farms have adopted mulberry-based diets, fresh or processed, for Manduca sexta, which introduces similar risks.
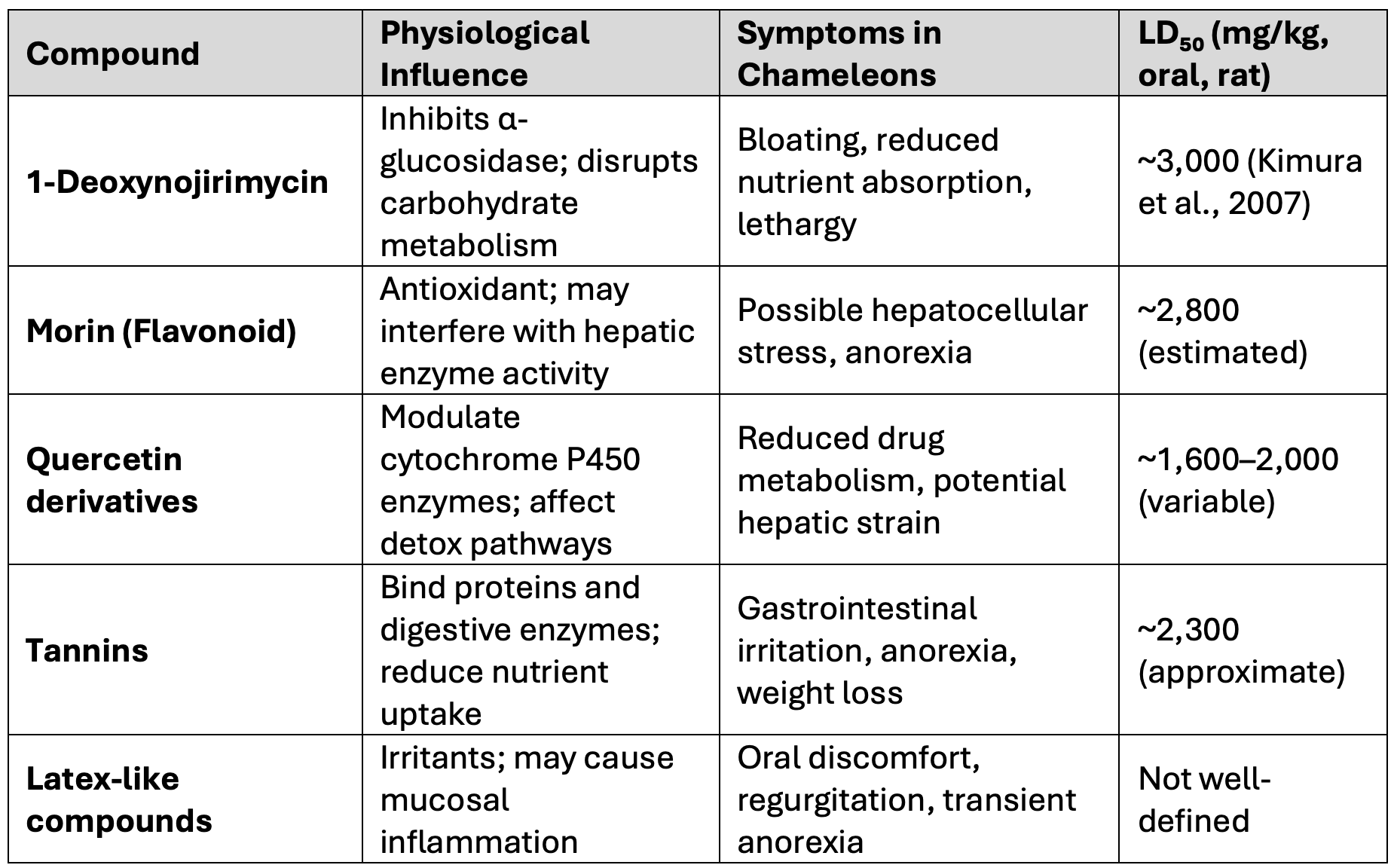
The primary phytochemicals retained in the gut content of silkworms include:
- 1-Deoxynojirimycin (DNJ) – a potent α-glucosidase inhibitor
- Flavonoids – including morin and quercetin derivatives
- Tannins – polyphenolic compounds with protein-binding properties
- Latex-like compounds – irritants present in mulberry sap
These compounds are not sequestered into silkworm tissue but may remain in the digestive tract if the larvae are consumed shortly after feeding. The following table summarizes their toxicological profiles:
Table: Mulberry-Derived Toxins in Silkworm Gut Content
Hidden Hazards: Seven Scientific Challenges in Feeding Hornworms and Silkworms to Chameleons
Introduction Hornworms and silkworms are often marketed as premium feeders, praised for their softness, hydration value, and ease of consumption. Yet beneath this convenience lies a complex web of biological risks. This section outlines seven critical concerns that are frequently overlooked in captive care protocols. Each chapter draws on verified scientific literature to expose the metabolic, anatomical, and behavioral consequences of relying on these insects as staple feeders. These are not minor caveats—they are foundational flaws that demand reevaluation of current practices.
1. Nutritional Imbalance and Calcium–Phosphorus Ratio
Hornworms (Manduca sexta) and silkworms (Bombyx mori) are commonly used as feeder insects in captive reptile husbandry, but their nutritional profiles present serious limitations. Most notably, both species exhibit calcium-to-phosphorus (Ca:P) ratios that fall well below the recommended threshold for insectivorous reptiles. Hornworms typically contain a Ca:P ratio of 1:3 to 1:5, while silkworms range from 1:2 to 1:4 (Mahanta et al. 2023; Finke 2002). The minimum recommended Ca:P ratio for reptiles is 2:1 to support proper skeletal development and prevent metabolic bone disease (Allen, Oftedal, and Horst 1996).
Inadequate calcium intake, especially when paired with excess phosphorus, leads to secondary nutritional hyperparathyroidism. This condition causes calcium to be leached from bone tissue, resulting in deformities, weakness, and neuromuscular dysfunction. Chameleons, which have high calcium demands during growth and reproduction, are particularly vulnerable. Without supplementation or dietary diversity, reliance on hornworms or silkworms can result in chronic mineral imbalance and irreversible pathology.
2. Digestive Load and Risk of Impaction
Hornworms grow rapidly and accumulate dense tissue and metabolic waste in their final instars. Their cuticle becomes increasingly resistant to enzymatic breakdown, especially when reared at high temperatures. Finke (2013) observed that late-stage hornworms may present a digestion challenge due to their leathery integument and high uric acid content. Silkworms, while softer, also accumulate silk proteins and chitin in later stages, which can resist breakdown in the reptile gut.
Impaction occurs when undigested material obstructs the gastrointestinal tract. In chameleons, this risk is amplified by low hydration, suboptimal basking temperatures, and slow digestive transit. Juveniles and gravid females are especially susceptible due to reduced gut motility and increased metabolic demands. Feeding large, late-instar hornworms without hydration control or temperature support can result in gastrointestinal stasis, requiring veterinary intervention.
3. Peristaltic Obstruction and Intestinal Anatomy
Chameleons possess one of the shortest intestinal tracts among vertebrates relative to body size. This anatomical adaptation reflects their insectivorous diet and high metabolic turnover. Short intestines require mechanical stimulation from roughage to maintain peristaltic movement. In natural conditions, this is achieved through ingestion of prey with chitinous exoskeletons, which provide bulk and texture.
Hornworms and silkworms, once digested, become amorphous and liquid in consistency. This lack of structural integrity means that the chyme may fail to stimulate peristalsis effectively. Stagnation of liquefied material in the intestinal lumen can lead to anaerobic bacterial proliferation. Species such as Clostridium perfringens and Enterobacter cloacae produce endotoxins and enterotoxins that can enter systemic circulation, causing vomiting, hepatic stress, and metabolic acidosis (Ratajczak et al. 2013). In severe cases, septicemia and organ failure may occur.
Chameleons rely on peristaltic efficiency to clear waste and maintain gut health. Feeding exclusively soft-bodied insects without compensatory fiber or hydration control increases the risk of peristaltic failure and microbial intoxication.
4. Gut-Loading Limitations and Micronutrient Delivery
Gut-loading is a critical strategy in reptile nutrition, allowing feeder insects to deliver essential micronutrients prior to consumption. However, hornworms and silkworms are poor candidates for gut-loading due to their limited nutrient retention. Finke (2003) demonstrated that hornworms fed calcium-rich chow retained minimal calcium in their tissues, with most excreted rapidly. Silkworms similarly exhibit low bioavailability of supplemented vitamins and minerals.
Fat-soluble vitamins such as A and D3 are particularly difficult to deliver through these insects. Without external supplementation, chameleons may develop deficiencies that impair vision, immune function, and calcium metabolism. In contrast, crickets and roaches retain and transfer nutrients more effectively, making them superior for gut-loading protocols (Allen and Oftedal 1994). Reliance on hornworms or silkworms without dusting or dietary rotation compromises micronutrient intake and long-term health.
5. Behavioral Conditioning and Dietary Monotony
Chameleons exhibit selective feeding behavior influenced by prey texture, movement, and moisture content. Hornworms and silkworms, being soft-bodied and highly hydrated, can condition chameleons to reject other feeder types. This behavioral shift leads to dietary monotony and increased risk of nutritional imbalance.
Repeated feeding of hornworms has been associated with reduced acceptance of crickets, roaches, and other staple insects (Finke 2002). The high palatability and moisture content of hornworms may override natural foraging instincts, especially in captive environments with limited prey diversity. Silkworms, while less aggressive in conditioning, still pose similar risks when used excessively.
Dietary monotony undermines ecological validity and increases the likelihood of micronutrient deficiencies. Chameleons evolved to consume a wide range of arthropods, each contributing distinct nutritional profiles. Overreliance on any single feeder type, particularly one with known limitations, compromises behavioral enrichment and physiological resilience.
6. Risk of Hyperhydration and Disruption of Homeostasis
Hornworms and silkworms contain moisture levels exceeding 80 percent by mass. While beneficial in dehydrated reptiles, this poses a risk when hydration protocols are not adjusted. Chameleons are adapted to intermittent water intake and precise osmoregulation. Excessive water intake can lead to hyperhydration and systemic disruption.
Hyperhydration dilutes extracellular and intracellular solutes, leading to:
Electrolyte imbalance: Hyponatremia and hypokalemia impair neuromuscular function and cardiac rhythm (Allen et al. 1996).
Enzymatic inefficiency: Dilution of intracellular fluids reduces enzyme activity, impairing digestion and thermoregulation.
Cellular rupture: Osmotic influx causes swelling and lysis, particularly in renal and hepatic tissues.
Renal overload: Increased glomerular filtration demands can lead to nephropathy or urate retention.
Behavioral dysregulation: Lethargy, reduced feeding drive, and impaired thermoregulation may result from systemic discomfort.
These risks are magnified when hornworms or silkworms are used as primary feeders without adjusting misting frequency, droplet size, or ambient humidity. Chronic overhydration can reduce lifespan and compromise organ function.
7. Economic and Husbandry Constraints
Silkworms and hornworms present logistical challenges that limit their practicality as staple feeders. Silkworms require mulberry-based chow, which must be refrigerated and prepared daily. Their sensitivity to temperature, humidity, and sanitation makes them labor-intensive to rear. Hornworms produce large volumes of waste and require frequent cleaning to prevent ammonia buildup and bacterial proliferation.
From an economic standpoint, both feeders are significantly more expensive than crickets or roaches. Their short shelf life and specialized dietary needs increase operational costs for breeders and keepers. For large-scale reptile facilities or multi-species collections, reliance on hornworms or silkworms is financially unsustainable.
Limited availability in some regions restricts access for hobbyists and professionals. This creates disparities in husbandry quality and forces reliance on suboptimal alternatives. Ethical husbandry demands that feeder choices be biologically appropriate and logistically feasible. Hornworms and silkworms, while valuable as occasional supplements, fail to meet these criteria as primary food sources.
Final Conclusions and Practical Recommendations for Chameleon Keepers
The use of hornworms (Manduca sexta) and silkworms (Bombyx mori) in captive chameleon diets has become widespread due to their soft texture, high moisture content, and ease of consumption. However, this convenience conceals a complex array of biological, nutritional, and toxicological risks that must be critically addressed. The preceding chapters have demonstrated that these insects, while useful in specific contexts, are fundamentally misaligned with the physiological and ecological needs of chameleons.
Hornworms, particularly when wild-caught, pose significant danger due to their capacity to sequester plant-derived alkaloids and glycoalkaloids. Even commercially bred specimens carry risks related to artificial dye exposure, microbial contamination, and nutritional imbalance. Silkworms, though less chemically defended, contain bioactive compounds such as 1-deoxynojirimycin (DNJ) and tannins that impair digestive enzyme function and nutrient absorption. These effects are magnified when larvae are fed immediately prior to being offered to reptiles. To minimize exposure, silkworms should be fasted for 12 to 24 hours before feeding.
Both species exhibit poor calcium-to-phosphorus ratios, low gut-loading efficiency, and excessive moisture content. These traits contribute to metabolic bone disease, peristaltic failure, and hyperhydration when used without dietary rotation or hydration control. Chameleons, with their short intestinal tracts and precise osmoregulatory systems, are particularly vulnerable to these disruptions. Feeding large quantities of soft-bodied larvae without compensatory roughage or environmental adjustment can result in impaction, microbial intoxication, and systemic metabolic stress.
From an ecological standpoint, the reliance on caterpillars as staple feeders is biologically incongruent. In the wild, chameleons rarely consume larvae due to their cryptic behavior and chemical defenses. Instead, adult moths and butterflies are more frequently targeted, offering higher encounter rates and superior nutritional profiles. During metamorphosis, both Manduca sexta and Bombyx mori undergo water resorption and lipid accumulation, resulting in adult forms with lower moisture, more structural chitin, and metabolically useful macronutrients (Nijhout, 1994). The wings and exoskeletal projections of adult moths stimulate peristalsis and aid digestive transit, reducing the risk of gastrointestinal stasis.
Therefore, the recommendation is clear: do not rely on hornworms and silkworms as staple feeders. Their use should be limited, strategically timed, and supported by rigorous sourcing from hygienic, transparent breeding operations. When possible, consider transitioning to adult moths, which offer improved nutritional balance and reduced toxicological risk. Supplement all soft-bodied feeders with calcium, monitor hydration protocols, and maintain dietary diversity to support long-term health.
Feeding practices must be guided not by convenience or market availability, but by biological compatibility and scientific evidence. The cost of neglecting these principles is paid in organ stress, behavioral decline, and shortened lifespan. Responsible husbandry demands nothing less than precision, transparency, and respect for the physiological integrity of the animals in our care.
Key Takeaways for Chameleon Keepers
Bad Ca:P Ratio – Hornworms and silkworms have too much phosphorus, not enough calcium.
Impaction Risk – Late-stage worms are harder to digest and can block the gut.
Peristalsis Failure – Liquefied worms don't move well through the short chameleon intestine.
Toxin Build-Up – Silkworms fed recently may carry DNJ and tannins that harm digestion.
Hyperhydration Danger – Too much water from worms can wreck electrolyte balance and cell function.
Poor Gut-Loading – These worms don't hold nutrients well; dusting is mandatory.
Behavioral Conditioning – Chameleons may reject other feeders after too many soft-bodied ones.
Moths Are Better – Adult moths have more nutrients, better texture, and match wild diet.
Source Matters – Only use worms from clean, transparent breeders with known diets.
Rotate, Don't Rely – Use hornworms and silkworms sparingly, never as staples.
References
Allen, M. E., and Oftedal, O. T. 1994. "Gut-loading feeder insects with calcium: A survey of current practices." Herpetological Review 25(2):51–53.
Allen, M. E., Oftedal, O. T., and Horst, M. N. 1996. "Essential nutrients in reptile husbandry." Journal of Zoo and Wildlife Medicine 27(1):2–15.
Benli, P. P. 2024. "Responses of Fishes, Amphibians, and Reptiles to Neonicotinoids." In Neonicotinoids in the Environment, pp. 75–90. Springer. https://doi.org/10.1007/978-3-031-45343-4_6
Borzelleca, J. F., Olson, J. W., and Reno, F. E. 1990. "Lifetime toxicity/carcinogenicity study of FD&C Blue No. 1 in rats and mice." Food and Chemical Toxicology 28(9):707–715.
EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). 2010. "Scientific Opinion on the re-evaluation of Brilliant Blue FCF (E 133) as a food additive." EFSA Journal 8(11):1884.
Finke, M. D. 2002. "Complete nutrient composition of commercially raised insects." Zoo Biology 21(3):269–285.
Finke, M. D. 2003. "Gut-loading to enhance the nutrient content of insects as food for reptiles: A review." Herpetological Review 34(3):196–202.
Finke, M. D. 2013a. "A comparison of the nutritional value of four species of commercially available feeder insects." Journal of Exotic Pet Medicine 22(4):332–337. https://doi.org/10.1053/j.jepm.2013.09.004
Finke, M. D. 2013b. "Complete nutrient content of four species of feeder insects." Zoo Biology 32(1):27–36. https://doi.org/10.1002/zoo.21012
Grill, C. P., and Götz, K. G. 1992. "Biliverdin-binding proteins in insect epidermis: Function and distribution." Journal of Comparative Physiology B 162(1):1–7.
Hanioka, N., Tanaka-Kagawa, T., and Jinno, H. 2008. "Genetic polymorphisms of cytochrome P450 enzymes in reptiles: Implications for xenobiotic metabolism." Comparative Biochemistry and Physiology Part C 147(2):119–125.
Kimura, M., Suzuki, M., and Yamaguchi, T. 2007. "Inhibitory effects of mulberry leaf extract on glycosidase activity and postprandial hyperglycemia." Journal of Nutritional Science and Vitaminology 53(3):287–290. https://doi.org/10.3177/jnsv.53.287
Kumar, P., Pandit, S. S., Steppuhn, A., and Baldwin, I. T. 2014. "Natural history-driven, plant-mediated RNAi-based study reveals the function of nicotine in plant-herbivore interactions." Proceedings of the National Academy of Sciences USA 111(4):1245–1252. https://doi.org/10.1073/pnas.1314848111
Mahanta, D. K., Komal, J., Samal, I., et al. 2023. "Nutritional aspects and dietary benefits of silkworms: Current scenario and future outlook." Frontiers in Nutrition 10:1121508. https://doi.org/10.3389/fnut.2023.1121508
Nijhout, H. F. 1994. Insect Hormones. Princeton, NJ: Princeton University Press. 280 pp.
Ratajczak, M. Z., et al. 2013. "The impact of synthetic food dyes on gut microbiota and immune function." Journal of Immunotoxicology 10(4):385–393.
Sharma, R., and Gautam, N. 2015. "Food color additives: Toxicity, health risks and regulatory issues." International Journal of Pharmaceutical Sciences and Research 6(2):472–480.
Tolley, K. A., and Herrel, A., eds. 2013. The Biology of Chameleons. Berkeley, CA: University of California Press. 288 pp.
Yildiz, D., et al. 2013. "Solanine and tomatine toxicity: A review of the mechanisms and clinical implications." Toxicology Letters 216(3):197–202. https://doi.org/10.1016/j.toxlet.2012.12.021

