Plantar Lesions in Chameleons: Etiology, Environmental Triggers, and Husbandry Implications
By Petr Nečas
Plantar Lesions in Chameleons: Etiology, Environmental Triggers, and Husbandry Implications
Abstract
Plantar lesions in chameleons are increasingly recognized as a multifactorial condition affecting both wild and captive populations. These disorders arise from mechanical trauma, environmental stressors, toxic plant exposure, and husbandry deficiencies. This article synthesizes clinical observations, histopathological findings, biomechanical stress factors, and veterinary case studies to provide a comprehensive overview of plantar pathology in chameleons. A husbandry risk matrix is proposed to guide preventive strategies, and evidence-based protocols are outlined to improve welfare outcomes.
Keywords chameleons, pododermatitis, plantar lesions, histopathology, husbandry, toxic plants, biomechanical stress, reptile dermatology
Introduction: Chameleon Foot Morphology and Function
Chameleons possess a distinctive digital arrangement known as chamaeleodactylous morphology, characterized by fused digits forming opposable bundles. These pincer-like structures enable precise grasping of branches and are supported by smooth, sensitive plantar surfaces equipped with microstructures analogous to gecko setae (Diaz and Trainor 2015). This adaptation facilitates arboreal locomotion but renders the feet vulnerable to abrasion, moisture retention, and chemical irritation.
Observations in Wild Populations
Although less common in the wild, plantar lesions have been documented in several species due to contact with introduced or invasive plants:
- Furcifer petteri,
in Montaigne de Ambre, Madagascar: a male found sitting on the introduced Mexican Weeping Pine, Pinus patula (originally from Mexico), with sap-contaminated soles unable to shed, inflamed and partly teared to bloody lesions. The tree is well known to bleed its quite liquid sap, which is very gluey initially and hardens to form more or less sticky fields on the trung and branches.
- Kinyongia multituberculata
in Lushoto, Tanzania: several specimens found on the Caribbean Copper Plant, Euphorbia cotinifolia (originally from Latin and Southern America), with soles and parts of the bodies contaminated by the white latex-sap. The animals were lethargic and might have suffered the toxic influence of the sap. The plant is very fragile and richly bleeds latex/sap from wounds. Even several tens of grams weighing chameleons break the leaves and their terminal branches easily.
- Kinyongia multituberculata
in Lushoto, W Usambara, Tanzania: several specimens found with bleeding lesions on soles at the grounds of Lushoto Executive Hotel, where the walls and big trees are overgrown with the introduced Creeping Fig, Ficus pumila (originally from East Asia) and Common Ivy, Hedera helix, (originally from Europe and Western Asia). The Creeping fig is known to have a toxic sap, the Ivy is famous by being rich in Oxalates forming very sharp crystals.
- Kinyongia matschiei
in Amani, E Usambara, Tanzania: a male specimen found in a bamboo plantation (probably introduced Fargesia sp. from central Asia) with bleeding soles. The sharp edged serrated leaves can cause cuts in heavy specimens that slip on the smooth leaves, moreover, sharp microfibers loosened from the stem can stick in the soles and cause contamination, inflammations.
- Chamaeleo calyptratus
in Miami and and in Fort Myers, Florida, USA (introduced feral populations) found on the invasive introduced Brazilian Peppertree, Schinus terebinthifolius (originally from Southern America) with sap-contaminated soles. The sticky sap is known to be able to cause contact dermatitis and in general the plant is toxic.
These cases document the risk posed by non-native flora, especially those with toxic sap, trichomes, or abrasive surfaces.
Etiology and Pathogenesis of Plantar Lesions
Plantar lesions in chameleons typically begin as erythema or pressure points and may progress to ulceration, necrosis, or osteomyelitis if untreated. The pathogenesis often involves:
Mechanical trauma from abrasive substrates or wire mesh
Excessive humidity, especially during nocturnal inactivity
Restricted movement due to enclosure size or feeding practices
Overweight and rapid growth, increasing pressure on plantar surfaces
Secondary infections by opportunistic bacteria such as Staphylococcus aureus, Aeromonas spp., and Pseudomonas spp. (Palmeiro and Roberts 2013; Vetlexicon 2023)
Histopathology and Microbiology of Plantar Lesions
Histological analysis of plantar lesions reveals epidermal necrosis, bacterial colonization, and inflammatory infiltration. Common pathogens include Staphylococcus aureus, Pseudomonas spp., and Aeromonas spp., often isolated from ulcerated or necrotic tissue (Palmeiro and Roberts 2013; Wellehan and Divers 2019). Fungal invasion by Fusarium, Paecilomyces, and Aspergillus species has also been documented in systemic and cutaneous infections (Jacobson et al. 2000). Inflammatory profiles show heterophilic infiltration, macrophage aggregation, and occasional granuloma formation. In advanced cases, radiographic imaging may reveal joint involvement or bone degradation (Samour et al. 2021).
Husbandry Risk Matrix
Environmental factors strongly influence lesion severity. Captive environments often amplify risk due to artificial materials and poor moisture control. The following matrix summarizes key risks:
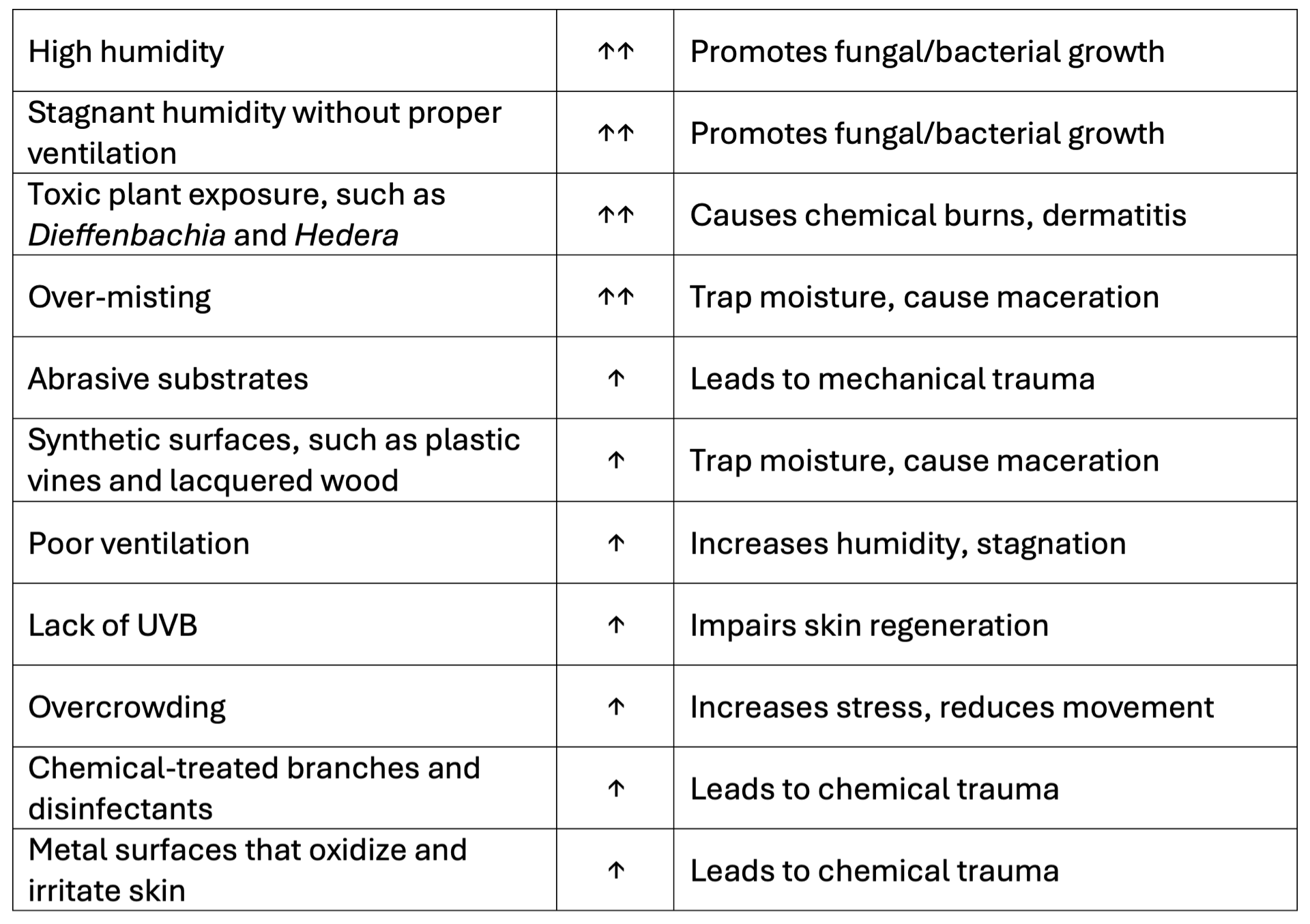
Modified and adapted from Zwart (2001) and Divers (2020).
Symptoms range from mild discoloration to exposed musculature and systemic infection. Chameleons often conceal pain, delaying detection until lesions are advanced.
Biomechanical Stress and Overweight
Pododermatitis is well-documented in other reptiles and birds, including falcons and tortoises. Similar grading systems apply, ranging from superficial hyperemia to septic arthritis and osteomyelitis (Zsivanovits et al. 2021; Vetlexicon 2023). In chameleons, biomechanical stress from overweight or limb amputation may lead to compensatory pressure and contralateral lesions (Madcham.de 2019). Captive chameleons often suffer from limited movement and excess body mass, contributing to pressure necrosis. Sedentary behavior reduces circulation to extremities, while overweight individuals exert disproportionate force on plantar surfaces. Analogous studies in falcons show similar progression from hyperemia to osteomyelitis (Zsivanovits et al. 2021). Jordaan et al. (2019) documented fire-induced trauma in reptiles, highlighting how environmental stress exacerbates tissue vulnerability.
Plant Toxicity and Dermatitis
Contact with toxic or abrasive plants is a major contributor to plantar lesions. Species such as Dieffenbachia, Ficus pumila, Philodendron, Euphorbia cotinifolia and diverse Bamboo species produce irritant sap or microfibers that damage reptilian skin (Vivarium Vibes 2022; Pond Informer 2022). Reptiles lack evolved defenses against these non-native plants, making them especially susceptible. Reptiles Magazine (2011) published a comprehensive list of ornamental plants known to be toxic to reptiles, either through ingestion or contact. Several ornamental and invasive plants have been documented to cause skin problems in reptiles, particularly chameleons, through contact with their soles. These plants either exude toxic sap, produce sharp microfibers, or harbor surface compounds that lead to dermatological reactions upon touch. Here's a curated selection of the most relevant entries from that list, focusing on those linked to dermatitis or systemic toxicity:
- Dieffenbachia species, commonly known as dumb cane, contain calcium oxalate crystals that can cause severe blistering and chemical burns when the sap contacts the skin.
- Philodendron and Caladium are similarly equipped with irritants that may induce redness, ulceration, and swelling.
- Ficus species, including Ficus pumila, are often cited in veterinary reports for causing localized inflammation and dermatitis due to their milky latex sap.
- Euphorbia cotinifolia, another species producing latex, is particularly notorious for causing skin ulceration and even plantar necrosis.
Other plants such as Epipremnum aureum (commonly called pothos) and Hedera helix (English ivy) tend to cause milder irritation but can still result in itching or inflammation on contact.
- Schinus terebinthifolius (Brazilian peppertree), although less common in captivity, contains urushiol-like compounds that may trigger swelling and lesions.
Mechanical abrasion also plays a role. Bamboo species like Fargesia, though popular in terrarium decor, possess serrated leaf edges and rigid fibers that can cut into soft plantar tissue.
Conifers produces sticky resin that can solidify around the feet and interfere with normal shedding, ultimately causing microlesions.
- Primula species, particularly those with hairy leaves, are known allergens that can lead to blistering and skin irritation in reptiles sensitive to plant hairs or surface oils.
Exposure to any of these plants in moist conditions can intensify the damage due to increased sap absorption and microbial colonization. They represent a clear risk for chameleon pododermatitis, especially when present in enclosures or habitats where reptiles rest or climb.
Preventive Husbandry Protocols
Divers (2020) and Mans (2017) provide detailed husbandry standards for arboreal reptiles.
Enclosure Design
Vertical space is essential: height should be at least 2–3× the reptile's total length.
Use secure, textured branches for climbing and resting.
Avoid glass-only enclosures; they cause stress and poor insulation.
Provide multiple hides at different elevations and temperatures.
Ensure proper ventilation without compromising humidity.
Temperature and Heating
Maintain a thermal gradient: basking zone at one end, cooler retreat at the other.
Use radiant heat sources (e.g., incandescent bulbs) positioned above basking sites.
Avoid "hot rocks" and in-cage heaters due to burn risk.
Nighttime temperatures should drop slightly to mimic natural cycles.
Monitor temperatures with multiple thermometers, especially near basking zones.
Lighting and UVB Exposure
Diurnal arboreal reptiles require UVB (290–300 nm) for vitamin D₃ synthesis.
Use high-quality UVB-emitting bulbs (e.g., mercury vapor, fluorescent tubes).
Position bulbs within 30 cm of basking sites; replace every 9–12 months.
Avoid filtered light through glass or plastic, which blocks UVB.
Provide 12-hour photoperiods to simulate natural day/night cycles.
Humidity and Hydration
Maintain relative humidity between 60–90%, depending on species.
Use misting systems, foggers, or live plants to regulate humidity.
Avoid reducing ventilation to increase humidity—this causes respiratory disease.
Offer water droplets on leaves or drip systems for species that don't drink from bowls.
Substrate and Furnishings
Use absorbent, non-toxic substrates like coconut fiber or paper-based bedding.
Avoid sand, gravel, or aromatic wood chips (e.g., cedar).
Include climbing structures, foliage, and textured surfaces to aid shedding.
Clean enclosures with mild soap and water; avoid phenolic disinfectants.
Nutrition and Feeding
Identify species-specific dietary needs: insectivorous, herbivorous, or omnivorous.
Gut-load insects and dust with calcium supplements before feeding.
Avoid feeding directly on substrate to prevent impaction.
Monitor feeding behavior and adjust based on age, season, and health.
Quarantine and Recordkeeping
Quarantine new arrivals for 3–6 months.
Keep detailed records of diet, health changes, treatments, and environmental adjustments.
Behavioral Considerations
Arboreal reptiles are often territorial; house singly unless breeding.
Minimize handling to reduce stress.
Observe for signs of thermal seeking, dehydration, or dysecdysis.
Veterinary Case Studies
A captive Furcifer lateralis developed bilateral plantar necrosis after prolonged exposure to synthetic perches and excessive humidity. Bacterial culture revealed Staphylococcus aureus. Treatment included topical silver sulfadiazine and systemic enrofloxacin. Recovery was complete following substrate replacement and humidity correction (Fraser and Girling 2019).
A juvenile C. calyptratus housed in a plastic-furnished enclosure developed ulcerative pododermatitis. Lesions progressed to deep tissue involvement. Surgical debridement was performed under anesthesia, followed by meloxicam for analgesia and topical antiseptics. Environmental modification led to full recovery (Meyer and Selleri 2019).
A male F. pardalis presented with advanced inflammation and abscess formation on the plantar surface following palm injury. The lesion was exacerbated by restricted movement and overweight. Necrotic tissue was surgically removed, and systemic antibiotics were administered. Healing occurred over several weeks with improved husbandry (Madcham.de 2019).
A large adult C. parsonii developed pressure-induced pododermatitis due to overweight and limited exercise. Initial signs included redness and swelling of the footpads. Despite appearing to walk normally, the animal exhibited dangling limbs and belly-resting behavior. Early intervention with weight reduction, dry overnight conditions, and soft branches prevented progression to abscessing (Madcham.de 2019).
A subadult F. oustaleti housed in a poorly ventilated enclosure developed plantar irritation and fungal colonization. Swabs revealed Fusarium spp. and Pseudomonas aeruginosa. Treatment involved topical antifungals, systemic antibiotics, and improved airflow. The case highlighted the role of microclimate in lesion development (Palmeiro and Roberts 2013).
These cases emphasize the importance of early detection, species-specific care, and environmental correction. If you'd like, I can integrate these into your article or build a lesion progression chart based on species and severity.
Conclusions
Plantar lesions in chameleons are a preventable yet serious condition rooted in environmental and husbandry factors. By integrating clinical observations, comparative pathology, and ecological awareness, keepers and veterinarians can reduce incidence and improve outcomes. Further research into biomechanical stress, plant toxicity, and microbiome interactions will enhance our understanding of reptilian dermatology.
References
Diaz, R. E., and P. A. Trainor. 2015. Hand/foot splitting and the 're-evolution' of mesopodial skeletal elements during the evolution and radiation of chameleons. BMC Evolutionary Biology 15:184.
Divers, S. J. 2020. Management and Husbandry of Reptiles. MSD Veterinary Manual. https://www.msdvetmanual.com/exotic-and-laboratory-animals/reptiles/management-and-husbandry-of-reptiles
Fraser, M. A., and S. J. Girling. 2019. Dermatology. In BSAVA Manual of Reptiles, 3rd ed., edited by S. J. Girling and P. Raiti, 257–272. BSAVA, UK.
Girling, S. J., and P. Raiti. 2019. BSAVA Manual of Reptiles. 3rd ed. BSAVA, UK.
Jacobson, E. R., J. L. Cheatwood, and L. K. Maxwell. 2000. Mycotic diseases of reptiles. Seminars in Avian and Exotic Pet Medicine 9(2):94–101.
Jordaan, P. R., A. C. van der Goot, H. P. Muller, and J. C. A. Steyl. 2019. Fire-induced reptile mortality following a management burn on Lapalala Wilderness, South Africa. Herpetology Notes 12:1173–1177.
Madcham.de. . 2019. Pododermatitis in Chameleons. Retrieved from https://www.madcham.de/en/pododermatitis/
Mans, C. 2017. Reptiles: Managing Husbandry Related Disorders. ISVMA Proceedings. https://www.isvma.org/wp-content/uploads/2017/10/Reptiles_Managing_HUSBANDRY_Related_Disorders.pdf
Meyer, J., and P. Selleri. 2019. Dermatology – Shell. In Mader's Reptile and Amphibian Medicine and Surgery, 3rd ed., edited by S. J. Divers and S. J. Stahl, 712–720. Elsevier, USA.
Palmeiro, B. S., and H. Roberts. 2013. Clinical approach to dermatologic disease in exotic animals. Veterinary Clinics of North America: Exotic Animal Practice 16(3):523–577.
Pond Informer. 2022. Toxic Plants for Reptiles. https://pondinformer.com/toxic-plants-for-reptiles/
Reptiles Magazine. 2011. List of Plants That Can Be Toxic to Reptiles. https://reptilesmagazine.com/list-of-plants-that-can-be-toxic-to-reptiles/
Vivarium Vibes. 2022. 15 Toxic Plants to Keep Out of Your Reptile's Terrarium. https://vivariumvibes.com/toxic-plants-to-keep-out-of-your-reptiles-terrarium/
Wellehan, J. F. X., and S. J. Divers. 2019. Bacteriology. In Mader's Reptile and Amphibian Medicine and Surgery, 3rd ed., edited by S. J. Divers and S. J. Stahl, 235–246. Elsevier, USA.
Zsivanovits, P., J. Samour, and M. B. Wernick. 2021. Therapeutic Management of Pododermatitis in Falcon Medicine: Historical and Modern Perspective. Archives of Veterinary and Animal Sciences 3(1). https://doi.org/10.5281/zenodo.5145208

